
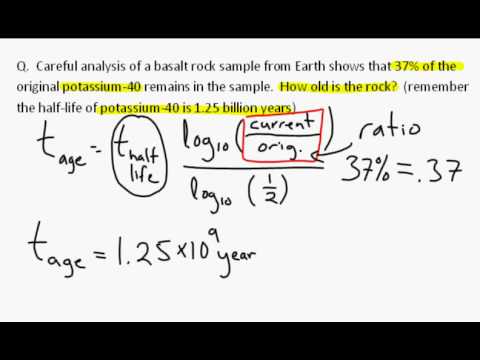
After another 5730 years, we would have 0.25 kilograms of carbon-14.
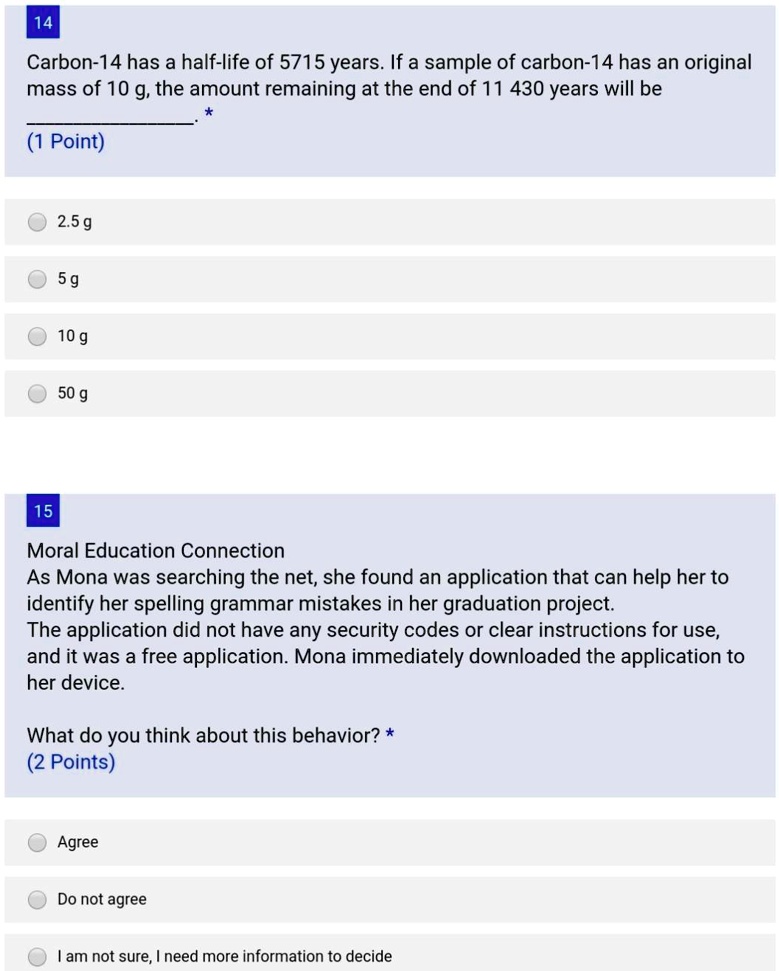
The variable t is how much time has elapsed, and t 2 1 is the half-life of the substance.Ī simple example is if we start with 1 kilogram of carbon-14, then after 5730 years we would have 0.5 kilograms of carbon-14.
The variable N 0 is how much of your substance you started with at time 0. The variable N t is the amount of carbon-14 you have left at time t. The formula used for calculating half-life is N t = N 0 ( 2 1 ) t 2 1 t . The half-life of carbon-14 is 5730 years. Calculating the half-life of carbon-14 is done by using a mathematical formula that relates to the amount of carbon-14 remaining in a sample with the age of the sample.


 0 kommentar(er)
0 kommentar(er)
